Ethics Review Committee of the Faculty of Science
Do you want to know if you should submit your research to the Ethics Committee? The Ethics Committee of the Faculty of Science is always available to review scientific research from employees of the Faculty. Feel free to contact us for advice.
When to contact the Ethics Committee?
- When the outcome of your research can lead to misuse or dual use.
- When you collect, process or analyze people’s personal data.
- When your research involves procedures or substances that can cause harm to humans, the environment or to animals.
- When you are unsure about the ethical consequences of your research proposal.
- When you use genetic resources or biological samples.
- Whenever you are in doubt on ethical issues concerning your research.
When to contact the METC-LDD
- When you do (bio)medical research on human subjects or material
When to contact the Central Authority for Scientific Prodecures on Animals (CCD)
- When your research involves experiments on animals
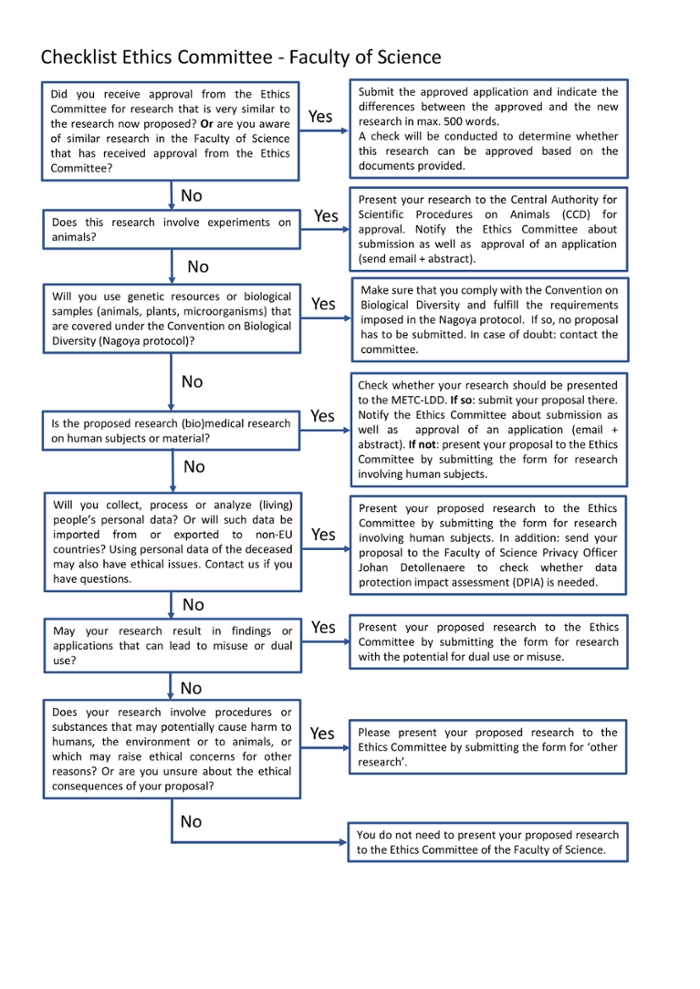
What happens when I submit my research?
If you submit your research to the committee, various procedures are possible. Check the flowchart below to see which procedure applies to you.
There is also a downloadable version with clickable links available.
More information
- Prof. Suzan Verberne, LIACS, chair
- Dr. Olga Gadyatskaya, LIACS
- Dr. Pedro Rodrigues Dos Santos Russo, STRW
- Dr. Vadim Cheianov, LION
- Prof. Hans Slabbekoorn, IBL
- Dr. Roxanne Kieltyka, LIC
Secretary: Monique Leemkuil
The Ethics Review Committee’s task is to assess proposed scientific research performed by (or under the responsibility of) employees of the Faculty of Science, against criteria of ethically responsible scientific practice and to advise researchers on ethical issues in scientific research.
The Committee assesses research proposals on the basis of the following codes of conduct and sources:
- Royal Netherlands Academy of Arts and Sciences (KNAW)
Royal Netherlands Academy of Arts and Sciences (2012). Zorgvuldig en integer omgaan met wetenschappelijke onderzoeksgegevens. Advies. The Hague: KNAW. /
Royal Netherlands Academy of Arts and Sciences (2013). Responsible Research Data Management. Advisory Report. The Hague: KNAW. - Royal Netherlands Academy of Arts and Sciences (2013). Vertrouwen in wetenschap. The Hague: KNAW.
This document includes an English summary, in which its title is translated as “Trust in Science”. - Association of Universities in the Netherlands (VSNU)
Netherlands Code of Conduct for Research Integrity (2018). - Dutch Data Protection Authority (APG)
Information about the General Data Protection Regulation (GDPR) and its implementation in practice. - Dutch Medical Research (Human Subjects) Act (WMO)
In the case of medical research involving human beings, the research must first be submitted to the Central Committee on Research Involving Human Subjects (CCMO).
Regulations of the Ethics Review Committee of the Faculty of Science
Below you can find some example files for consent forms, agreements or data storage.
- Example of how to store personal data
- Example informed consent form Consent Parent or Guardian (Dutch)
- Example informed consent form Consent participant (Dutch)
- Example Confidentiality agreement student
For further information and requests for testing, please send an e-mail to ethicscommittee@science.leidenuniv.nl, or contact secratary Monique Leemkuil.
